Independent Lab Tested for Filtration Efficiency




Product Details
Don't risk your safety with a counterfeit. Our KN95 masks are either FDA approved (see masks with Emergency Use Authorization or EUA description) and/or lab tested for GB2626-2006 compliance. Some of the compliance requirements include 95%+ filter efficiency to filter out at least 95% of particulates in the air with 0.3 microns or greater in size. These respirators would also be effective in filtering out ash particles to protect your health and lungs from wildfire smoky conditions. Cloth masks do not achieve the same level of filtration. According to the standards testing, our KN95 mask achieved 97%+ Filter Efficiency.
Our masks have gone through an additional quality assurance (earloop, liquid and verification of layers) testing with our onsite staff before the masks are shipped from the manufacturer’s warehouse abroad.
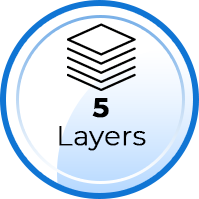
Our KN95 masks are made of 5 layers, which include: two (2) outer layers of non-woven fabrics for comfort and for filtering coarse particles, one (1) layer of heat sealing cotton (broad-spectrum antibacterial), and TWO (2) layers of melt-blown, ultra-fine, non-woven fibers which are highly effective with small particle filtration. A typical surgical mask has only 3 layers and some KN95 masks have 4 layers. Assuming the filter layers are of similar materials (melt-blown, non-woven fibers), a mask with 5 layers should provide higher Filtration Efficiency than those with 3 or 4 layers.
Our manufacturers are FDA registered and passed the lab testing for GB2626-2006 standards for KN95 designation, as well as certified for EN149:2001+A1:2009 standards related to CE Directives R2016/425 (Personal Protective Equipment). All certifications and registrations are posted on our site, providing complete transparency.


5 Layers - Cut and Separation Test Performed by TenMasks Team
Don't Risk Buying a Low Price Counterfeit!
* KN95 Masks with 5 Layers of Protection
* 95%+ Filter Efficiency
* FDA Approved (EUA) and/or FDA Registered and ECM Certified
* Lab Tests (Both Manufacturer AND Independent Lab) Reports Available

What's the difference
between "KN95" and "N95"?
KN95 is a Chinese standards designation similar to N95 (U.S. - NIOSH-42C FR84), FFP2 (European – EN149-2001), P2 (Australia & New Zealand – AS/NZ 1716:2012), and KF94 (Korea – KMOEL-2017-64). They are similar mainly for the Filter or Filtration Efficiency ratings of 95%+ (KN95) and 94%+ (others). However, they differ in terms of “Total Inward Leakage” (eg Air & particle leakage INTO the mask due to facial fit, breathing, and movements).
The non-NIOSH N95 standards (KN95, FFP2, P2 & KF94) allow for 0-8% Total Inward Leakage. The Total Inward Leakage is not part of the NIOSH certification standards since there are many factors that can impact the Total Inward Leakage rate such as the wearer's facial features; however, most of the 3M's N95 masks achieve less than 1% Total Inward Leakage due to their double-headband, tight fitting designs and therefore suitable for extremely high risk environment such as hospitals.
Given the scarcity of N95s, which should be ideally reserved for healthcare workers, the KN95s are ideal for both anyone in healthcare and/or the general public as they are a suitable equivalent to the N95s, according to the FDA's Emergency Use Authorization. The KN95s also offer superior protection (compared to cloth or surgical masks) against ash particles in smoky conditions. More information by the CDC on how to reduce exposure to wildfire smoke:
https://www.cdc.gov/disasters/covid-19/reduce_exposure_to_wildfire_smoke_covid-19.html
How to Identify Fake or Poor Quality Imports
Leakage Test – The layers of the KN95 masks are water proof. You can verify by pouring water inside the mask and observe whether there are leakages (even small droplets forming on the bottom) after 1 minute or longer.
Smell Test - Put on your mask and empty the contents of a pack of Sweet and Low on a flat surface. Try sniffing with the mask on and then off. You can still smell the saccharin with the mask on but only faintly.
Improper Labeling – Some manufacturers label their products with an FDA logo and claim that their products are FDA approved. FDA does not test these products nor approve any of the PPEs. Manufacturers can be registered with the FDA, but a submission of the products (for testing or even inspection) are not part of the registration process. The improper use of the FDA logo is prohibited. This is also very misleading to consumers, who would have a false sense of comfort when they believe the product is FDA approved.
Note: The mask will become unusable after the following test: Layers – Typically, a KN95 masks should have at least 4 layers, which can be observed by cutting the mask and separating the layers. Note: The creamy white, melt-blown, non-woven filter layers are very fine (super thin), so it would take some effort to separate them for a 5-layer masks.

